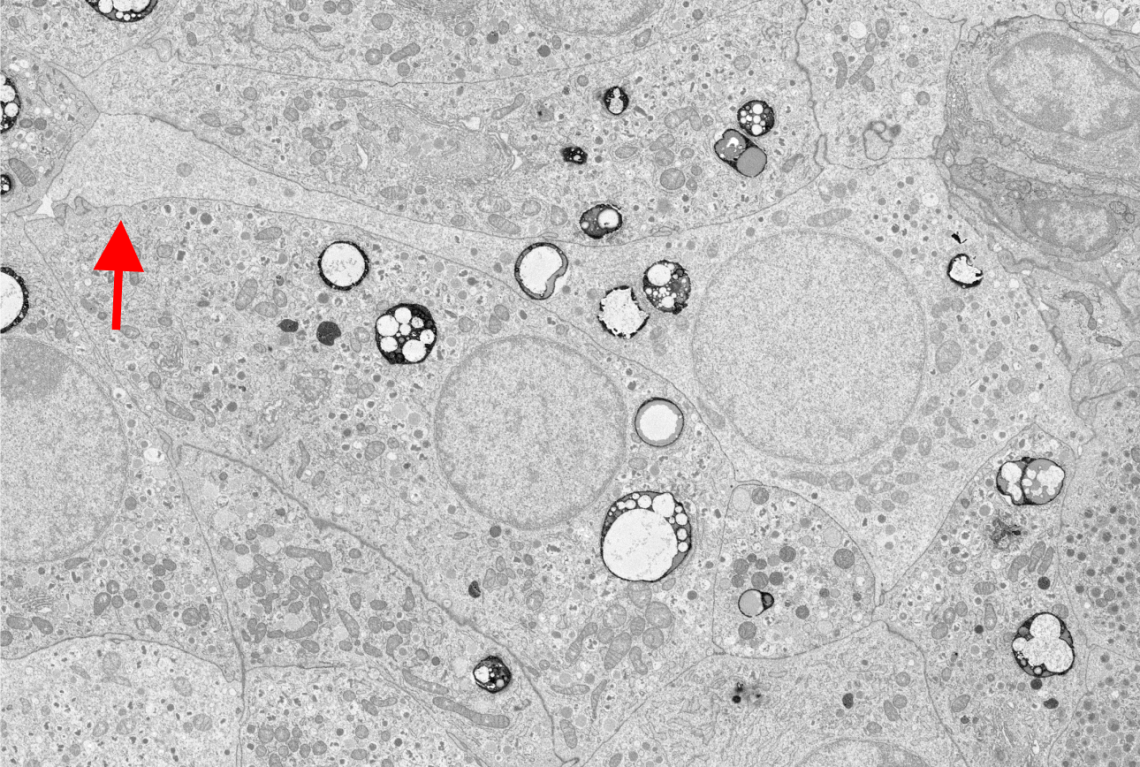
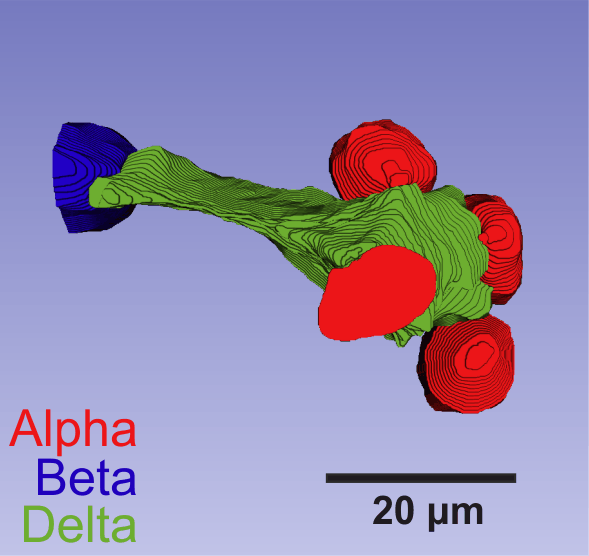
Human islet delta-cells exhibit long projections
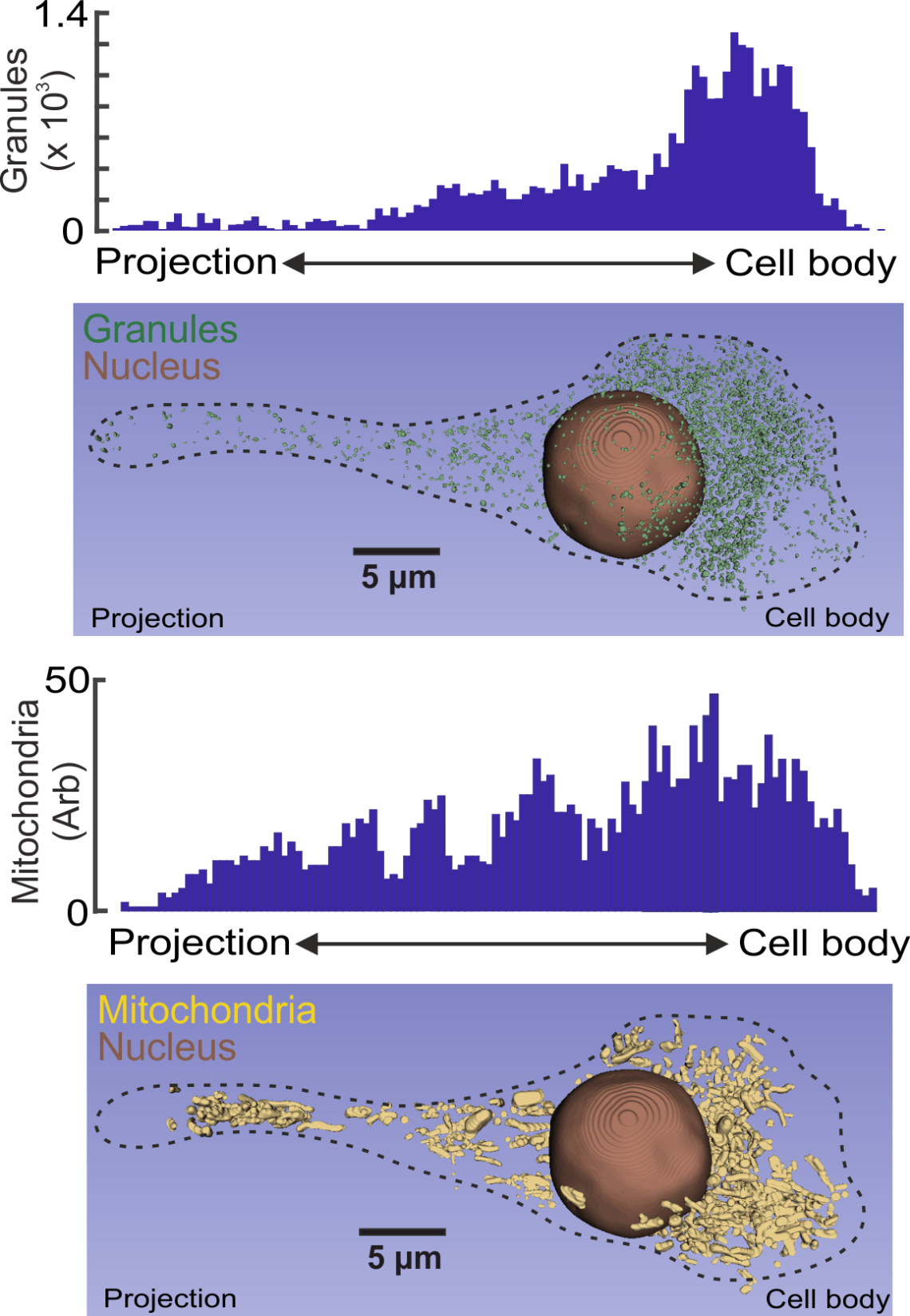
Granule and mitochondria distribution in a human delta-cell
Colleges
Linford Briant
PhD, MSci
Sir Henry Wellcome Postdoctoral Fellow & Junior Research Fellow at Trinity College
- Mathematical modeller
- Patch-clamp electrophysiologist
- Dynamic imaging
- Time-series analysis
Computational and experimental investigation of islet cells
The pancreatic islets contain beta-cells and alpha-cells, which are responsible for secreting two principle gluco-regulatory hormones; insulin and glucagon, respectively. However, they also contain delta-cells, a relatively sparse cell type that secretes somatostatin (SST). These cells have a complex morphology allowing them to establish an extensive communication network throughout the islet, despite their scarcity. Delta-cells are electrically excitable cells, and SST secretion is released in a glucose- and KATP-dependent manner. SST hyperpolarises the alpha-cell membrane and suppresses exocytosis. In this way, islet SST potently inhibits glucagon release.
Recent studies by myself and my colleagues has investigated the activity of delta-cells with optogenetics, electrophysiology and Ca2+ imaging, and revealed they are electrically coupled to beta-cells via gap junctions. This suggests that the delta-cell is more than just a paracrine inhibitor.
In my work, I aim to investigate delta-cell morphology, function, and the role of SST signalling for regulating islet hormonal output both in health and disease. I pay particular attention to the importance of this novel gap junction pathway, because it offers fresh insight into the contribution of delta-cells to the islet hormonal defects observed in both type 1 and type 2 diabetes. This reassessment of the role of the delta-cell is important as it may offer novel insights into how the physiology of this cell can be utilised to restore islet function in diabetes.
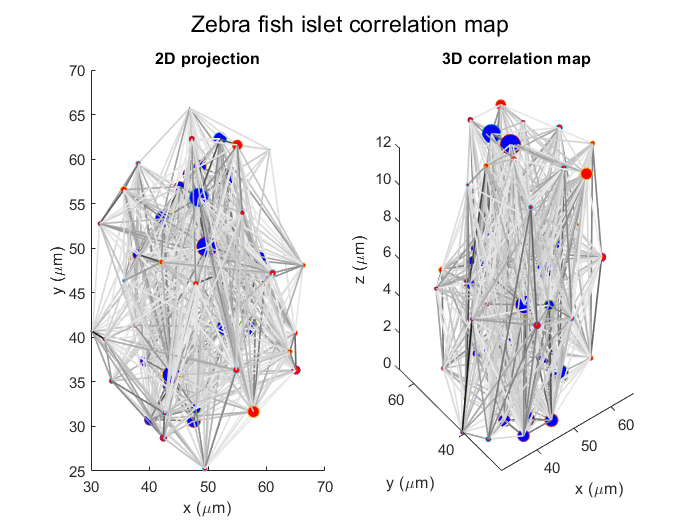
Computational tools to understand dynamic islet cell interactions in 3D
ORCID
0000-0003-3619-3177
Recent publications
-
Heterogenous impairment of α cell function in type 2 diabetes is linked to cell maturation state.
Journal article
Dai X-Q. et al, (2022), Cell Metab, 34, 256 - 268.e5
-
Gap junction coupling and islet delta-cell function in health and disease
Journal article
Miranda C. et al, (2022), Peptides, 147
-
Arginine-vasopressin mediates counter-regulatory glucagon release and is diminished in type 1 diabetes.
Journal article
Kim A. et al, (2021), Elife, 10
-
Arginine-vasopressin mediates counter-regulatory glucagon release and is diminished in type 1 diabetes
Journal article
Kim A. et al, (2021), eLife
-
The importance of islet delta-cell ATP-sensitive K(+)channel (K-ATP) function for somatostatin secretion and islet hormone balance
Conference paper
Hill TG. et al, (2021), DIABETOLOGIA, 64, 64 - 64
Human islet delta-cells exhibit long projections
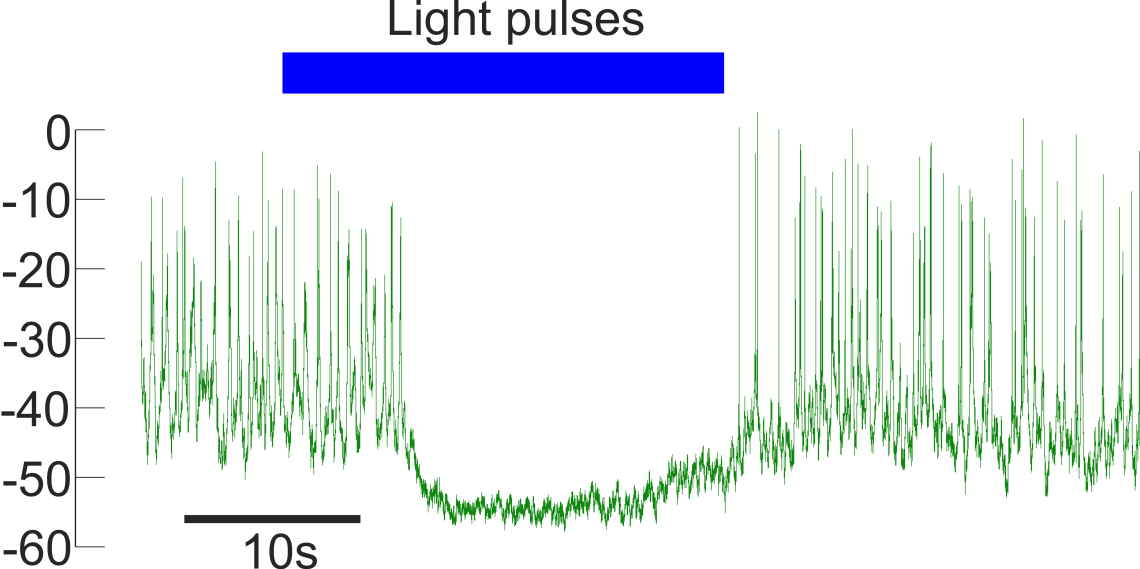
Opto-genetic silencing of alpha-cells